The finish of a Printed Circuit Board (PCB) in the competitive electronics manufacturing sector is more than a cosmetic consideration. It is a critical engineering choice determining the yield of the assembly, shelf life, and long-term reliability of the end product. Copper, though the core of the PCB, is also susceptible to the environment because it offers the conductive paths that carry power and signals.
The Underlying Problem: Oxidative Instability of Copper
Each PCB starts with Copper Clad Laminate (CCL), the fundamental block of circuit interconnection. Refined copper foil is a good conductor but extremely reactive. As soon as copper pads (small areas that will be soldered) are exposed, they start to oxidize almost immediately when exposed to oxygen and water.
Copper oxide forms a non-conductive film that compromises solderability. Solder does not bond to oxide well, and contributes to weak or dry joints. Moreover, resistivity levels significantly rise during oxidation, reducing electrical conductivity and potentially compromising the performance of the finished product. To avoid this, the soldering pad should be plated with an inert material such as gold, silver, or a protective coating of a chemical film (OSP) to ensure a good yield in later assembly processes.
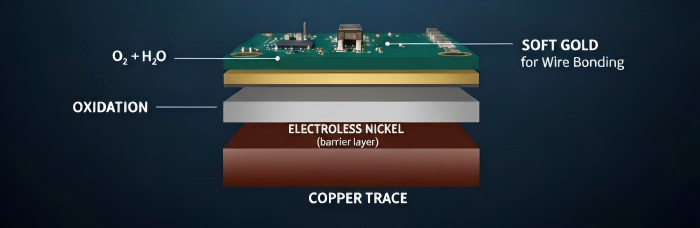
Outstanding Corrosion and Inertia
Gold is a noble metal that is chemically inactive. Gold does not oxidize or tarnish in air like copper or silver. This offers two significant benefits to PCB fabrication:
Long Shelf Life: A gold-plated board may last more than 12 months in a warehouse and remain perfectly solderable. Silver finishes, on the other hand, are tarnishable (to become silver sulfide), and copper-based finishes such as OSP may degrade within months, requiring immediate soldering upon plating.
Environmental Protection: Gold is an everlasting shield in industries, such as aerospace, medical, and undersea electronics, where boards are subjected to harsh climates of extreme humidity or salts. The golden fingers of an old board, years in service, will still be flashing and useful, but aluminum or iron would be rusted away to waste.
High-End Surface Flatness on Advanced Parts
Contemporary electronics are becoming miniaturized, necessitating the application of Fine-Pitch Surface Mount Technology (SMT) and Ball Grid Arrays (BGA). These mechanisms require a very smooth surface so that all contact points (often microscopic) are reliably in contact.
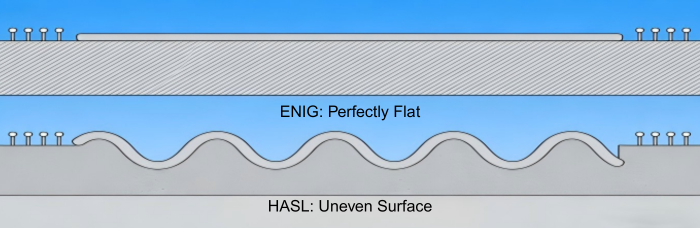
Conventional finishes such as HASL (Hot Air Solder Leveling) frequently leave behind irregular "mounds" or "meniscuses" of solder. Chemical gold processes, namely Electroless Nickel Immersion Gold (ENIG), offer an ideal planar surface, achieved by moving surface atoms. This guarantees proper placement of components and strong soldering connections, which is crucial in transporting digital signals quickly in computers and smartphones.
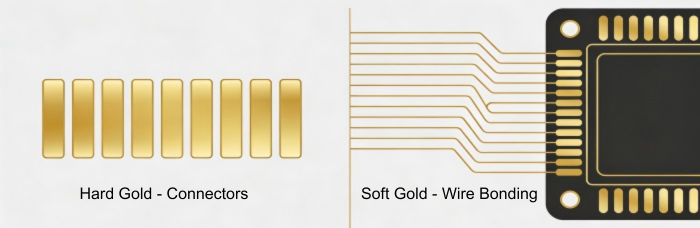
Professional Identification: Hard Gold vs. Soft Gold
There is no unified silver bullet. It may be adapted to particular mechanical and electrical requirements by varying the alloy content:
Hard Gold (Electrolytic Gold): Surface hardness is significantly enhanced by alloying gold with limited quantities of cobalt or nickel. This is critical to "Gold Fingers" (edge connectors) and connector shrapnel that needs to endure thousands of insertion or switching cycles without being worn out to the copper.
Soft Gold (Pure Gold): This is the plating of pure gold without alloys. COB (Chip on Board) applications and aluminum wire bonding are especially problematic. Its versatility enables robust, trustworthy bonds among semiconductor chips and the PCB.
The Technical Excellence of ENIG and ENEPIG
Gold is not commonly used directly on copper in professional fabrication. Direct contact will result in a physical response, with electrons migrating and diffusing. To avoid this, it would need a "barrier layer" of nickel. That is why the process is technically called Electroplated Nickel Gold.
Electroless Nickel Plating: Nickel is coated to a depth of 3-6mm on the copper. This serves as an obstacle, inhibiting the mutual dialysis of copper and gold that otherwise would undermine the joint.
Immersion Gold Deposition: Over the nickel, a thin sheet (0.03-0.15mm) of gold is deposited.
ENIG offers great flatness and shelf life, but can sometimes have the black disk issue, in which the nickel layer oxidizes over time, compromising long-term performance. To address such risks, ENEPIG (with a Palladium layer added) is adopted as a universal finish in the most challenging applications.
Why Gold Is Better Than Silver and Copper
Gold (ENIG/Hard Gold) is the top finishes when comparing. Although it is capable of costing approximately 10% of a PCB, its inert nature and constant contacts render it an ideal choice in low-voltage, low-resistance applications. It is the default in spacecraft and satellite and high-end smartphones critical components.
Strong alternatives can be found in Immersion Silver when the aim is to save money without compromising connectivity or flatness. It finds extensive application in automotive, communication products, and high-speed signal designs. But silver has flaws in its growth rate, including tarnishing and voids in solder joints, which may affect long-term reliability relative to gold.
The lowest cost is Bare Copper (OSP). As an external finish, it provides little protection, yet it is the main material within the PCB (Copper Clad Laminate). It is primarily applied in consumer electronics with short lifecycle or assembled directly after the board is fabricated.
A reliable surface finish will determine the reliability of the board, regardless of whether you are designing a high-speed telecommunications hub or a resilient consumer wearable. Only gold offers ideal combination of high conductivity, extreme corrosion resistance, and physical flatness.
At PCBCart, we take pride in providing highly accurate boards that match the industry standards. In contemporary electronics, good enough is seldom good enough. The substance that enables technology to extend the performance frontiers is gold, and as such, the innovations of the present become the reliable ones of the future.














